(www.dicardiology.com: December 14, 2021) – The National Capital Consortium for Pediatric Device Innovation (NCC-PDI) announces five awardees chosen in its prestigious annual “Make Your Medical Device Pitch for Kids!” competition to share $150,000 in grant funding from the U.S. Food and Drug Administration (FDA). The FDA grant supports the advancement of pediatric medical devices. In an unprecedented decision, the competition judges determined that all five finalists were deserving of a grant award and recognition for the potential patient benefit and commercial viability of their innovations.
Consistent with its mission of addressing the most pressing pediatric device needs, this year’s competition, conducted by NCC-PDI partner MedTech Innovator, focused on innovations in electrophysiology devices that monitor and treat congenital heart disease (CHD) and arrhythmias in pediatric patients. The virtual pediatric pitch event was part of the 9th Annual Symposium on Pediatric Device Innovation.
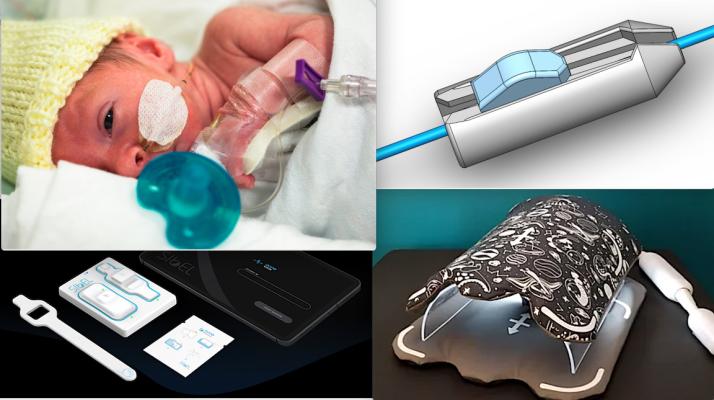
This year’s pediatric device innovation awardees are:
PeriTorq pediatric catheter grip • PeriCor (The Children’s Hospital at Montefiore, New York, N.Y.) PeriTorq, a catheter grip tool for use during pediatric cardiac interventional procedures — https://pericorllc.com/
• Karios Technologies (Charlottesville, Va.) Tissue Shield, a technology to prevent scar tissue formation (adhesions) on the heart after surgery.
Inkspace Imaging pediatric MRI coil. • Inkspace Imaging (Pleasanton, Calif.) a pediatric cardiac and vascular MRI coil — www.inkspaceimaging.com.
• Starlight Cardiovascular (San Diego, Calif.) Project Lifeline, a less-invasive way to maintain sufficient circulation in newborns with ductal-dependent circulation that increases safety, procedural success and ease of use. www.starlightcardio.com
Sibel Health ANNE One neonate wireless patient monitoring system.• Sibel (Niles, Ill.) ANNE One, ICU-grade wireless sensors for cardiopulmonary monitoring in neonates with congenital heart defects — www.sibelhealth.com/anne-one.
Congenital heart disease (CHD) affects six out of 1,000 babies born in the U.S. each year and is often complicated by arrhythmias, a condition where the heart beats too rapidly, too slowly or irregularly due to a misfiring of the body’s electrical impulses.
While the last decade brought great advances in technologies that improve the care of adult arrhythmias, pediatric patients have been left behind, with only five devices approved for use in children in the same period. As a result, pediatric specialists are often using off-label or improvised devices to treat pediatric arrhythmias, including in the smallest newborns.
“Recognizing this unmet need, NCC-PDI opened the challenge earlier this year to select companies to enter MedTech Innovator’s pediatric accelerator program, made possible by NCC-PDI. The five companies have immensely benefited from the accelerator program and are well-positioned to compete for funding. They have the potential to advance pediatric health and provide a greater standard of care for children living with CHD,” said Kolaleh Eskandanian, Ph.D., MBA, PMP, vice president and chief innovation officer at Children’s National Hospital and principal investigator of NCC-PDI. “For too long, the unique needs of children have been overlooked in pediatric device development. Thanks to the support of the FDA, we are able to build our challenge competitions around the direst unmet needs, which are determined through a thorough needs assessment and market analysis conducted to inform each request for proposal. The funding incentivizes pediatric innovation and helps more companies navigate the path to commercialization.”
NCC-PDI is one of five consortia in the FDA’s Pediatric Device Consortia Grant Program created to support the development and commercialization of medical devices for children, which lags significantly behind the progress of adult medical devices. NCC-PDI is led by the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Hospital and the A. James Clark School of Engineering at the University of Maryland, with support from partners MedTech Innovator, BioHealth Innovation and design firm Archimedic.
“Experts have long indicated that there are not sufficient technologies available on the market to support pediatric patients living with CHD. This overwhelming consensus motivated us to focus this year’s competition on reversing that trend,” explained William E. Bentley, Ph.D., distinguished university professor and director of the Robert E. Fischell Institute for Biomedical Devices at the University of Maryland. “We are delighted to unveil these winning innovations and wish awardees all the best as they take the next steps toward bringing these viable technologies to market.”
A pediatric accelerator program, powered by MedTech Innovator, is the consortium’s latest addition to a network of resources and experts that NCC-PDI provides in support of pediatric innovators. All five of this year’s competition finalists had an opportunity to participate in the year-long accelerator program.
“It has been a privilege getting to know and support the dedicated teams pioneering these transformative health solutions during MedTech Innovator’s accelerator program, which provides individualized mentorship and resources that companies need to successfully advance toward product commercialization” said Paul Grand, CEO of MedTech Innovator. “Pediatric devices are extremely challenging to bring to market, and we’re honored to leverage the world’s leading device ecosystem to ensure that these life-improving innovations successfully reach the children who so desperately need them.”
Eskandanian adds that supporting the progress of pediatric innovators is a key focus of the new Children’s National Research and Innovation Campus, a one-of-its-kind ecosystem that drives discoveries that save and improve the lives of children. On a nearly 12-acre portion of the former, historic Walter Reed Army Medical Center in Northwest Washington, D.C., Children’s National has combined its strengths with those of public and private partners, including industry, universities, federal agencies, start-up companies and academic medical centers, the campus provides a rich environment of public and private partners which, like the NCC-PDI network, will help bolster pediatric innovation and commercialization.
For more information:, https://childrensnational.org/research/explore, www.umd.edu, https://medtechinnovator.org/






Leave A Comment